9.5.2 Drinking water quality monitoring
Drinking water quality monitoring is used to provide assurance that the quality of drinking water in the distribution system, as supplied to the consumer, is meeting guideline values, agreed levels of service, and/or any regulatory requirements. It can provide an additional means of detecting any unrecognised problems that may be occurring upstream or within the distribution system, and can trigger the necessary corrective actions.
Drinking water quality monitoring cannot prevent unsafe water being supplied to consumers, as results are typically not available for days to weeks after collecting the sample, so that any corrective actions occur after the water has been supplied. Drinking water quality monitoring should not, therefore, be used in place of or as a substitute for operational monitoring.
As it is neither physically nor economically feasible to test for all drinking water quality characteristics equally, monitoring effort and resources should be directed at significant or ‘key’ characteristics – that is, those characteristics identified in the system-specific hazard identification and risk assessment process as likely to be present. These key characteristics require more frequent monitoring. Characteristics that the risk assessment shows are unlikely to be present, or pose a low risk, are monitored very infrequently, or may not need to be included in the drinking water quality monitoring program.
Generally, sampling and analysis are required most frequently to assure microbial safety and less often for chemical and radiological compounds. This is because of the acute and almost universal health risk posed by waterborne microbial pathogens, whereas the guideline values for most (but not all) chemical characteristics are based on lifetime exposure. In the absence of a specific event (e.g. spills, chemical overdosing at a treatment plant), episodes of chemical contamination that would constitute an acute health concern are rarer.
Sampling locations for drinking water quality monitoring
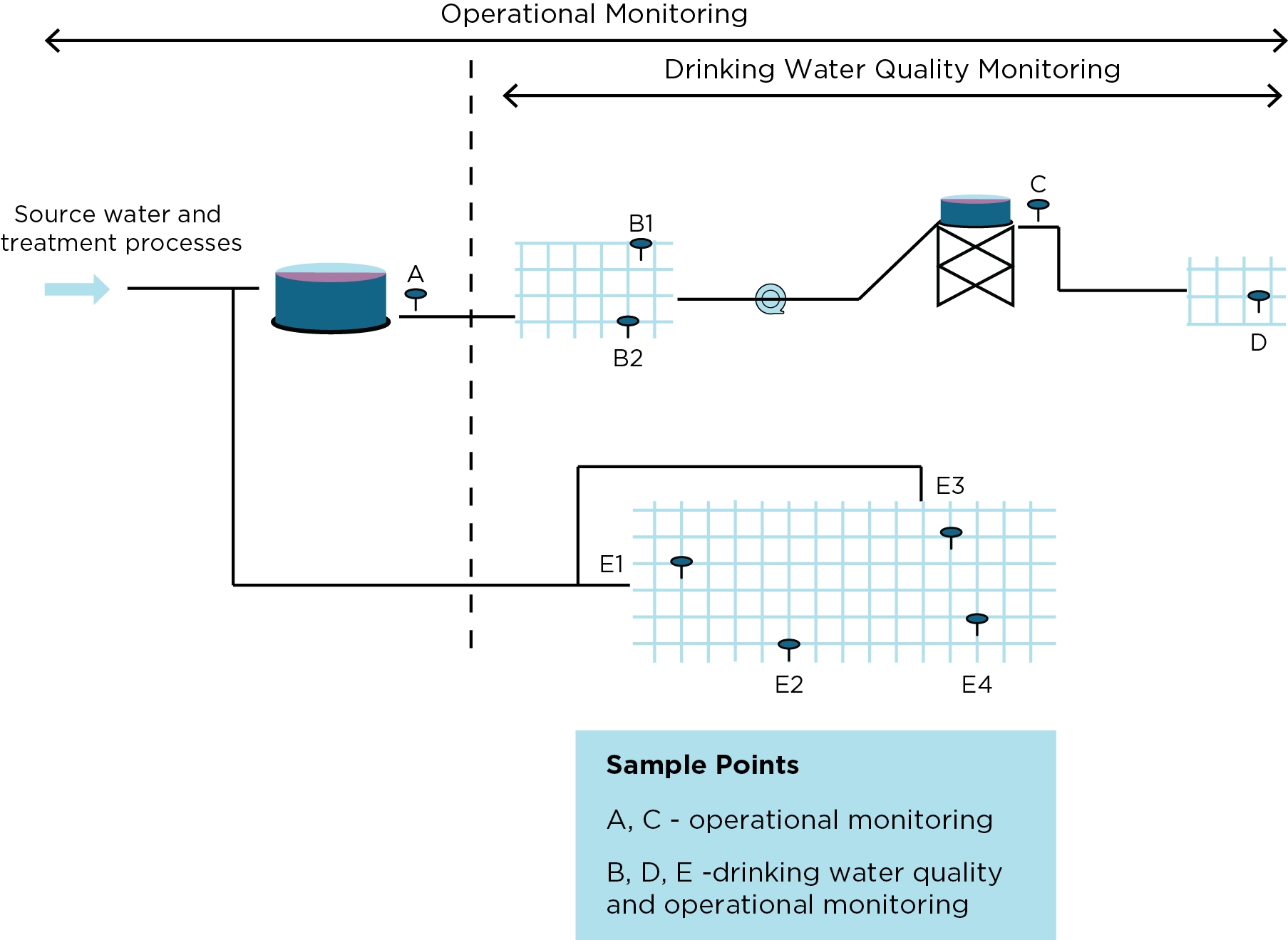
Drinking water quality monitoring confirms the final quality of water that is supplied to consumers. As such, it needs to be undertaken throughout the distribution system at points representative of the quality of water supplied to consumers’ properties (e.g. at or close to water meters).
The location and number of sampling points within a distribution system are determined by the complexity of the system. For purposes of management, monitoring and reporting, large and complex distribution systems should be divided into discrete water quality monitoring zones. These zones are typically:
supplied from a single source, and/or
hydraulically separated from other zones (single or multiple sources).
As the priority for monitoring drinking water quality is to confirm microbial safety, the design of the microbiological sampling program often dictates the location of sampling points. Sampling points are normally placed well into the distribution system to be representative of what most consumers have received. They should also be spread geographically to give coverage across the system or zone.
Circumstances where microbial quality has the potential to change within a distribution system need to be considered. This is most likely where the system is depressurised, increasing the chance for ingress (e.g. at a service tank). Sample points should, therefore, be included downstream of any tanks (often called a subzone) even though the source water may be unchanged.
Samples for physical and chemical quality monitoring can usually be taken from the sample points used for microbiological monitoring. Since physical and chemical quality monitoring requires many fewer samples in a given period, a decision must be made on whether to rotate sampling around all the sample points within a zone (providing an indication of performance across the zone) or to use only one or two fixed sample points (providing an opportunity to plot trends).
For chemical characteristics that are more stable and unaffected by the distribution system, sampling can occur at the entry point to the distribution system. For some characteristics that change across the distribution system e.g. trihalomethanes (THMs), additional monitoring from specific areas may be required to ensure the data collected are representative of all water supplied.
Operational monitoring such as chlorine residual monitoring is typically also carried out concurrently at these sample points, as well as at other strategic locations within the distribution system, such as entry points (e.g. outlets of service reservoirs/tanks), trunk mains, and dead ends.
Figure 9.3 provides an illustration of sampling locations within a typical distribution system and shows how operational monitoring and drinking water quality monitoring are integrated.
Figure 9.3 Example of sample locations in a typical distribution system
Microbial quality – sampling frequency
Routine monitoring for specific microbial pathogens is not recommended as it is usually complex, expensive and time-consuming, and may fail to detect their presence. Rather, the recommendation is to monitor for the microbial indicator bacterium E. coli as a marker for the presence of faecal contamination and the possible presence of microbial pathogens (see fact sheets on microbial indicators). Whilst there are limitations to the use of E. coli as an indicator of faecal contamination of water supplies (e.g. Cryptosporidium oocysts may survive chlorine disinfection and may be present in the absence of E. coli), it is currently the best verification indicator available for faecally related microbial quality.
The recommended minimum monitoring frequency for E. coli, based on World Health Organization recommendations (WHO 2008), is detailed in Table 9.4. Samples should be collected at points within the distribution system that are representative of the quality of water supplied to consumers.
Table 9.4 Recommended minimum frequency of E. coli monitoring
Samples that are representative of the quality of water supplied to consumers should be collected and analysed for E. coli at the following minimum frequency:
>100,000
Six samples per week per monitoring zone, plus one additional sample per month per monitoring zone for each 10,000 above 100,000
5,000–100,000
One sample per week per monitoring zone plus one additional sample per month per monitoring zone for each 5,000 above 5,000
1,000–5,000
One sample per week per monitoring zone (52 samples per year)
<1000
One sample per week per monitoring zone (52 samples per year). Where the water supply in this category is remote, the recommended sampling frequency needs to be balanced against the logistics of collecting the samples, the risk profile for the supply, and the risk mitigation processes that are operating on the supply. With remote water supply systems, regular physical inspections and operational monitoring are more beneficial to ensuring water quality than infrequent E. coli sampling.
Sampling frequency should be increased at times of flooding or emergency operations and following repair work or interruptions to supply.
The frequency of sampling should be increased during any significant environmental events or emergency operations, or following interruptions of supply or repair work. More frequent sampling should also occur at sample points where previous results have indicated potential problems. Operational monitoring such as disinfectant residuals, temperature and turbidity are often taken concurrently with E.coli to provide complementary evidence of system status and enhance interpretation of data.
The results of the E. coli monitoring program will not prevent unsafe water being supplied to consumers, and drinking water quality monitoring should not be substituted for or used in place of a well-constructed operational monitoring program. For systems serving small communities, regular physical inspections of the water supply system, and the monitoring of critical processes and activities, such as chlorination, yield more information than infrequent sampling (see Chapter 4).
Drinking water quality (non-microbial) – sampling frequency
Monitoring requirements for non-microbial characteristics will vary for each water supply system, depending on the key characteristics identified through water supply system analysis and risk assessment. In general, characteristics that pose a high level of risk require more frequent monitoring, while those posing a low risk require less monitoring. The closer the mean value of a characteristic is to the guideline value, and/or the greater its variability, the more frequent the monitoring needs to be. Those characteristics that are deemed, on the basis of a thorough analysis of the catchment and water supply system, unlikely to be present will typically require very infrequent monitoring, or no monitoring at all.
Table 9.5 provides a generic guide to monitoring frequency for drinking water quality characteristics. Monitoring frequencies and characteristics for individual systems should be adjusted as needed, based on the ongoing review of the water supply system and risk assessment.
Table 9.5 Generic frequencies for monitoring non-microbial drinking water quality as supplied to the consumer
Physical characteristics
pH
Temperature
Total dissolved solids¹
Colour
Turbidity
Dissolved oxygen
Hardness²
Taste and odour
¹ If reverse osmosis used, or there are known salinity issues, otherwise quarterly
² If water is treated for hardness
Water treatment related chemicals (if used)
Fluoride¹
Aluminium²
Chlorine
Copper (seasonal)
Any related organic contaminants, e.g. acrylamide, carbon tetrachloride, epichlorohydrin
¹ Will not require frequent monitoring if fluoridation is not carried out or if naturally occurring fluoride is either absent or at a level below the level for optimal fluoridation.
² Aluminium not likely to be present if no alum-based coagulant is used.
Disinfection byproducts (DBPs)
Trihalomethanes (THMs)¹𝄒²
Ammonia, nitrite, nitrate²
Bromate, formaldehyde³
Chlorite⁴
¹ Where chlorine or chloramine are used.
² Where chloramine is used.
³ Where ozone is used.
⁴ Where chlorine dioxide or liquid chlorine is used.
If detected at elevated concentrations, close to, or above guideline values, additional related DBPs should also be analysed.
Inorganics
Iron
Manganese
Arsenic, nitrate, fluoride, selenium, lead, mercury¹
Ammonia, cadmium, chromium, nickel, zinc, copper, hydrogen sulfide
Tin, silver beryllium, uranium, iodide, molybdenum, boron, barium
¹ Priority contaminants: quarterly sampling for groundwater sources, more frequent monitoring when detected at elevated concentrations; otherwise sampling reduced to annually, seasonally or event-related (e.g. storm events, reservoir turnover events).
Pesticides and organic toxicants
If detected or potential presence
If not detected
Monthly or quarterly sampling for pesticides/organic toxicants previously (or potentially) detected; seasonally annually, or event-related (e.g. storm events, spills) for other pesticides/organic toxicants.
Radiological
Radionuclides
New supplies should be assessed quarterly for one year, then every 2 years (groundwater) or 5 years (surface water). Increase frequency to quarterly if guideline screening levels exceeded.
Metals with potential for leaching
Bismuth, silicon, antimony, chromium, copper, nickel
Annual sampling, unless pipework material has been considered as part of the nominated sampling frequency.
Last updated