2.2: Radiological monitoring and assessment of performance (updated 2022)
Screening of water supplies
Radioactivity is present in the environment from natural and from anthropogenic (man-made) sources. Some radionuclides will be present in drinking water. The majority of these will be naturally occurring, including potassium-40 and the decay products from the uranium and thorium decay series as shown below.
Uranium decay series:
Thorium decay series:
Anthropogenic radionuclides that may be present, although in very low concentrations, include caesium-137 and strontium-90.
The process of identifying individual radionuclides and determining their concentration requires sophisticated and expensive analysis, which is normally not justified because concentrations in most circumstances are very low. A more practical approach is to use a screening procedure, where the total radioactivity present in the form of alpha and beta radiation is determined without regard to the identity of specific radionuclides. The use of a gross alpha and gross beta screening process is consistent with the approach adopted by the World Health Organization drinking water guidelines (WHO 2017).
The ‘screening’ values that are recommended for both gross alpha and gross beta activity are 0.5 becquerels per litre (Bq/L) for each. Potassium-40 is a natural beta emitter, which occurs in a fixed ratio to stable potassium. Potassium is an essential element for humans and does not accumulate in the body but is maintained at a constant level independent of intake. Therefore, the contribution of potassium-40 to beta activity is subtracted following a separate determination of total potassium. If the screening values are not exceeded (including the gross beta corrected for potassium-40), there is no need for further assessment.
If either or both screening values are exceeded, specific radionuclide measurements should be undertaken to calculate the radiation dose associated with drinking the water. It should be emphasised that the screening values are conservative and should never be treated as a limit, guideline value or as an indicative water quality target. Screening values are intended only as a practical, cost effective means to ascertain if further assessment of the radiological quality of the water supply is needed.
Dose assessment
The drinking water supply may be sourced from surface waters (e.g. reservoirs, rivers and dams), seawater desalination or from groundwaters. In the case of surface waters, the dose would be expected to be much less than 0.1 millisievert per year (mSv/year) (ARPANSA 2008). However, for groundwater, mineralisation of the water may lead to an increase in the radionuclide activity concentrations and therefore the dose could be higher than 0.1 mSv/year (ARPANSA 2008). Desalinated water is generally considered to have very low mineral content with the desalination processes such as reverse osmosis and distillation effective in the removal of radionuclides. Consideration should be given to the waste products generated in the desalination or other treatment processes used (WHO 2018).
In Australia, if the gross alpha and beta activity concentrations are both 0.5 Bq/L, a realistic worse case exposure scenario occurs when the gross activities are due entirely to radium-226 (an alpha emitter) and radium-228 (a beta emitter). Assuming that a gross alpha activity concentration of 0.5 Bq/L is attributable to radium-226 and a gross beta activity concentration of 0.5 Bq/L is attributable to radium-228, the calculated dose equates to 0.35 mSv/year.
Water that meets the screening values will therefore result in an annual dose of approximately one-third of the reference level of 1 mSv/year. Therefore the recommended screening values provide a good margin of safety against the reference level of 1 mSv/year.
The operational dose value of 0.3 mSv/year is a value where if exceeded, it may be reasonable to consider whether additional information is required to ensure the 1 mSv/year reference level is not exceeded. For example, repeat testing, further specific radionuclide analysis and/or an assessment of consumption patterns may be required. The 0.3 mSv/year operational dose value is also the point at which the screening value is generally exceeded, warranting further individual radionuclide analysis.
Australia has established an operational dose value that aligns with the WHO individual dose criterion (IDC). Australia has adopted an operational dose level of 0.3 mSv/year. This operational dose level takes into account naturally occurring radionuclides (notably radium) in Australian water supplies.[1] Naturally occurring radionuclides are more difficult to control and generally require specific radiochemical separations, which increases the costs and time required for the analysis when determining concentrations accurately at low levels. The decision to adopt an operational dose value of 0.3 mSv/year is consistent with the WHO advice for these situations.
If the screening values for gross alpha or gross beta activity are exceeded, further analysis is required to identify specific radionuclides and determine their activity concentrations. This may involve resampling the water if the volume of the original sample is inadequate to allow specific radionuclide analysis. Activity concentrations for the most common sources of emissions, radium-226 and radium-228, should be evaluated at this stage; however, other radionuclides may need to be considered. See the section Analytical methods for specific radionuclides in this Information Sheet. Further information on the evaluation of the radium activity concentrations can be found in the Radium fact sheet. The annual dose from ingestion of each radionuclide can be calculated using the method described in Chapter 7, Section 7.6.2.
If the sum of the annual doses from all radionuclides is less than the operational dose value of 0.3 mSv, no additional action is required and routine monitoring can continue. If the sum of the annual doses from all radionuclides exceeds the operational dose value of 0.3 mSv, it is not appropriate to rely on a single analysis to determine annual exposure. In this case water should be sampled at a frequency which should be agreed to by the relevant health authority or drinking water regulator.
These results should be reviewed as they become available, to ensure that there are no immediate problems. A final assessment of annual dose can be made when sufficient results representative of seasonal variations are available to characterise the radiological quality of the water supply. Average concentrations of each radionuclide can be used to calculate the annual dose.
Where a gross alpha and gross beta measurement has not been used in the screening or monitoring of the water supply, and instead a direct measurement of radium-226 and radium-228 isotopes is made, the 0.3 mSv/year operational dose value can serve as the equivalent screening value. Where a water supply exceeds the operational dose value, and a full assessment of the water supply has not already been undertaken, it may be reasonable to consider whether additional information is required to ensure that the 1 mSv/year reference level has not been exceeded e.g. repeat testing, further specific radionuclide analysis and/or an assessment of local consumption patterns.
The 0.3 mSv/year operational dose value should only be applied in decisions around monitoring. It is not a value at which intervention is expected to occur or to be initiated.
Operational response
The operational response will depend on the gross alpha and gross beta results or the estimated annual dose determined by the sum of the contribution from each radionuclide present in the water.
If the screening values have not been exceeded or the total annual dose is less than 0.3 mSv, the operational dose value has not been exceeded and routine monitoring can be maintained.
If the total annual dose lies between 0.3 and 1.0 mSv, the reference level has not been exceeded; however, further assessment and evaluation may be required. This may include sampling at a higher frequency along with an assessment of local water consumption habits to determine if protective measures should be considered.
If, with ongoing sampling, the annual dose repeatedly exceeds 1 mSv, protective measures should be considered. The water service provider and the relevant health authority or drinking water regulator should assess the results and examine options to reduce the levels of exposure. Water supply providers should consider operational changes that can be implemented at minimal cost to reduce the annual exposure. For example, where possible water could be taken preferentially from sources with the lowest radionuclide concentrations.
An annual dose that exceeds 10 mSv is unacceptable for drinking water and immediate protective measures should be taken to reduce the dose to below the reference level of 1 mSv/year.
The monitoring and assessment process is illustrated in Figure IS2.2.1 and recommendations on the operational response, based on dose, are presented in Table IS2.2.1.
Figure IS2.2.1 Flowchart showing how to determine whether the radiological quality of drinking water complies with the Guidelines
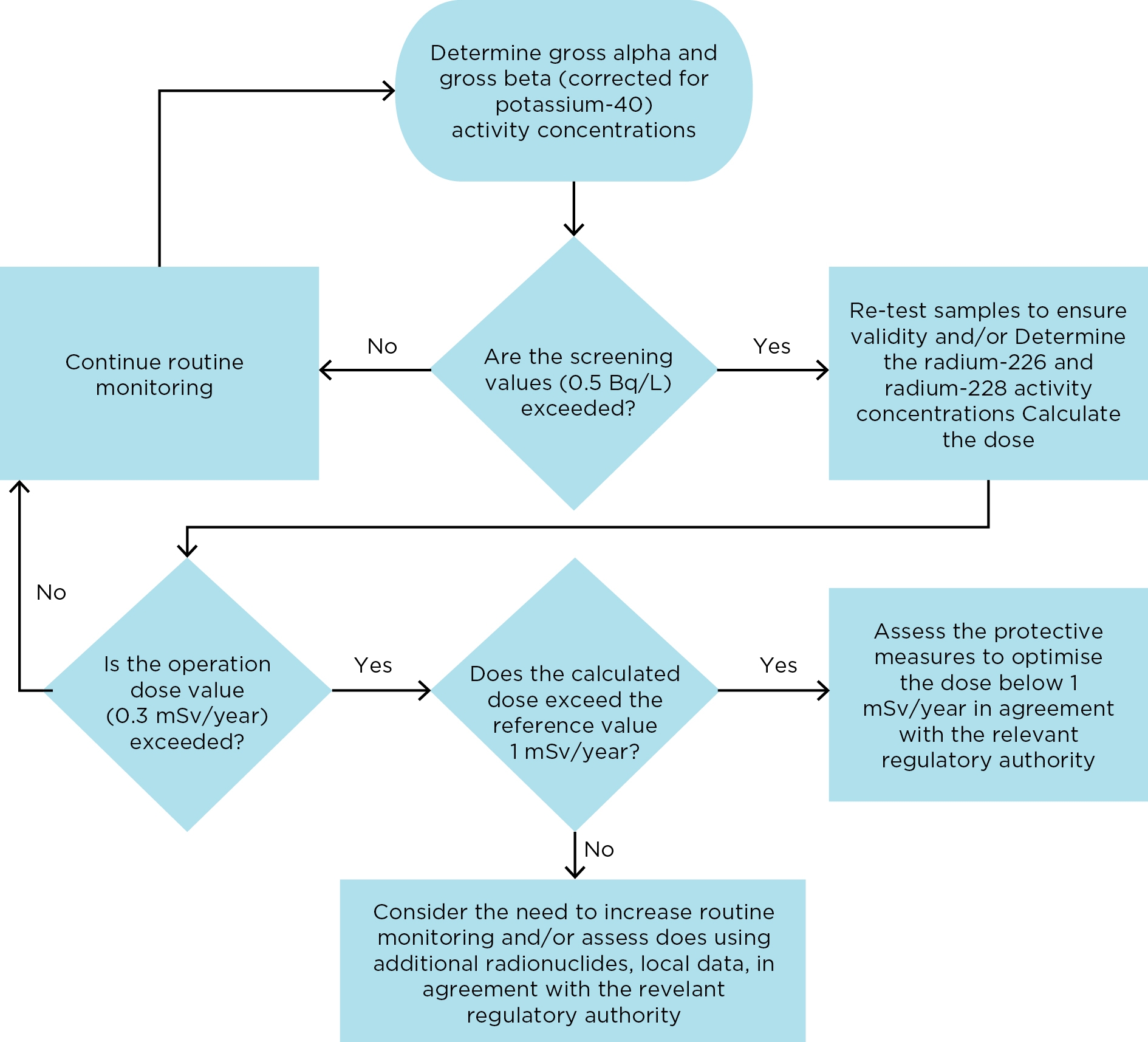
Table IS2.2.1 Summary of operational responses
<0.3
Gross alpha and gross beta screening values (corrected for potassium-40) and/or the operational dose value are not exceeded. Continue routine monitoring.
0.3-1
Evaluate dose and if required, perform assessments based on local conditions.
Consider the need to increase the frequency of monitoring in agreement with the relevant health authorities or drinking water regulators based on if the operational dose value is exceeded.
1-10
Consult with relevant health authorities or drinking water regulators.
Assess in detail possible protective measures e.g. remedial/protective actions, taking into account potential cost-effectiveness of actions.
Implement appropriate remedial/protective measures on the basis of the cost-benefit evaluation.
> 10
Water not suitable for consumption on the basis of radioactivity levels.
Consult with relevant health authorities or drinking water regulators.
Intervention is expected and protective measures must be taken to reduce doses to below the reference level of 1 mSv/year.
Methods of analysis
Gross alpha and beta activity concentration
For analysis of drinking water for gross alpha and beta activity two common methods of analysis are used:
evaporation of a known volume of the sample to dryness and measurement of the activity concentration of the residue. As alpha radiation is easily absorbed within a thin layer of solid material, the reliability and sensitivity of the method for alpha determination may be degraded in samples with a high content of total dissolved solids
evaporation of a known volume of the sample, the addition of a scintillation cocktail and measurement by liquid scintillation counting.
Standard methods (or validated methods) should be used to determine concentrations of gross alpha and beta activities. Table IS2.2.2 lists some recommended standard methods from the International Organization for Standardization (ISO), the American Public Health Association (APHA) and ASTM International (ASTM).
Table IS2.2.2 Recommended methods for the analysis of gross alpha and beta activities in drinking water
ISO 9696 Gross alpha (2017)
Evaporation
Proportional counting, alpha spectrometry
ISO 9697 Gross beta (2015)
Evaporation
Proportional counting
ISO 11704 Gross alpha and gross beta (2018)
Evaporation
Liquid scintillation counting
APHA 7110B Gross alpha and gross beta (2017)
Evaporation
Proportional counting
APHA 7110C Gross alpha (2017)
Co-precipitation
Proportional counting, alpha counting
APHA 7110D Gross alpha and gross beta (2017)
Evaporation
Liquid scintillation counting
ASTM D7283 (2017)
Evaporation
Liquid scintillation counting
# The determination of gross beta activity using any of the evaporation methods in Table IS2.2.2 includes the contribution from potassium-40. An additional analysis of total potassium is therefore required to correct for the potassium-40 contribution to the gross beta activity concentration.
* The detection limit is dependent on sample volume, counting time and the analytical method. It is recommended that the detection limit is < 20% of the screening values.
Limitations of the gross alpha and gross beta methods include:
the results for gross alpha and gross beta will be dependent on the method used and may be dependent on the radionuclides used for the calibration of the measurement equipment.
some beta emitting radionuclides are not detected by the gross alpha and gross beta method. These include tritium and volatile radionuclides such as radioactive iodine. These radionuclides are not expected to be present in drinking water supplies at levels that could be a health concern.
the activity concentrations of some low energy beta emitters such as lead-210 and radium-228 may be underestimated depending on the method of detection.
samples with high total dissolve solids (TDS) may interfere with some methods of analysis. The co-precipitation method is the most suitable for samples with a high TDS.
Analytical methods for specific radionuclides
If the screening values have been exceeded, the identification and analysis of individual radionuclides is required. In Australia, the presence of anthropogenic radionuclides in drinking water is unlikely and therefore the investigation should focus primarily on naturally occurring radionuclides. Based on the radiological health risk and the radionuclide abundance in Australian waters, the following should be analysed: radium-228, radium-226, polonium-210, lead-210, uranium-234 and uranium-238 (ARPANSA 2008, Kleinschmidt 2011, Walsh 2014). It is important to note that the guidelines for exposure to uranium isotopes are based on their chemical toxicity.
The uranium and thorium decay chains contain a number of other short-lived radionuclides that, if present, may contribute to the gross alpha and gross beta activity concentrations. It is important to note that due to the losses of gaseous radon isotopes in the screening measurements, and the properties of ingrowth and decay for the naturally occurring radionuclides that may be present in the sample, activity concentrations of the individual radionuclides may not sum to equal the gross alpha or gross beta activity concentrations.
Refer to the fact sheets for information on analysis of specific radionuclides. Fact sheets are available for: Specific alpha and beta emitting radionuclides, Radium (radium-226 and radium-228), Radon-222 and Uranium.
Sample handling and pretreatment
Water samples should be pretreated to prevent significant losses of radionuclides from solution following collection and in transit to a laboratory. Delays in sample analysis should be minimised.
Details of appropriate procedures for the handling of water samples, including suitable containers and pretreatment methods, are described in the relevant Australian/New Zealand Standards AS/NZS 5667.1, AS/NZS 5667.5 and AS/NZS5667.11 (AS/NZS 1998). Calmet et al. (2013) have compiled a list of ISO standards related to test methods for radioactivity monitoring of water.
Analytical methods for potassium-40
It is impractical to use a radioactive measurement technique to determine the concentration of potassium-40 in a water sample. The ratio of potassium-40 to stable potassium is fixed, therefore chemical analysis for potassium is recommended. The activity due to potassium-40 can then be calculated using a factor of 0.0276 Bq of beta activity per milligram of potassium.
Sampling frequency
New water supplies and those not previously sampled should be sampled often enough to characterise the radiological quality of the water supply and to assess any seasonal variation in radionuclide concentrations. Quarterly sampling over the first year should provide sufficient data to establish a baseline. More frequent sampling may be necessary if the water source displays variable screening results. Once the radiological quality of a supply has been established and shown to meet the screening values and/or the operational dose level, sampling for routine monitoring can be less frequent – every two years for groundwater supplies, every five years for surface water supplies.
Where screening is omitted in place of performing analysis of specific radionuclides, the 0.3 mSv/year operational dose can be used to determine the case for continued routine monitoring. For water supplies approaching the reference level of 1.0 mSv/year, consultation with the relevant regulatory authority may be required in establishing the frequency of an ongoing monitoring program. Ongoing monitoring should consider a graded approach based on the water source (surface water vs groundwater), the variability of radionuclide concentrations and the availability of previous data.
Reporting of results
The analytical results for each sample should contain the following information:
sample identifying code or information
reference date and time for the reported results (e.g. sample collection date)
identification of the standard analytical method used, or a brief description of any non-standard method used
the activity concentrations with an estimate of the total uncertainty
a minimum detectable concentration.
The estimation of the total uncertainty of the reported result should include the contributions from all the parameters within the analytical method (i.e. counting and other random and systematic uncertainties).
Footnotes
Extract from WHO’s Management of Radioactivity in Drinking-water (WHO 2018). An operational dose value (IDC) of “0.1 mSv/year is appropriate for most countries where groundwater supplies with elevated levels of naturally occurring radionuclides are not present. Where there are elevated levels of naturally occurring radioactivity in groundwater and minimal options for alternative water sources or water treatment, a value higher than 0.1 mSv/year, but generally less than the BSS (IAEA 2014) reference level of 1 mSv/year, may be appropriate for the affected population groups.”
References
APHA, AWWA, WEF (American Public Health Association, American Water Works Association, Water Environment Federation) (2017). 7110 B Evaporation Method for Gross Alpha-Beta.
APHA, AWWA, WEF (American Public Health Association, American Water Works Association, Water Environment Federation) (2017). 7110 C Coprecipitation Method for Gross Alpha-Beta Radioactivity in Drinking Water.
APHA, AWWA, WEF (American Public Health Association, American Water Works Association, Water Environment Federation) (2017). 7110 D Liquid Scintillation Spectroscopic Method for Gross Alpha-Beta.
ARPANSA (Australian Radiation Protection and Nuclear Safety Agency) (2008). The Radioactive Content of Some Australian Drinking Waters. Technical Report Series No 148.
AS/NZS (Australia/New Zealand Standard) (1998). AS/NZS 5667.1:1998 Water quality – Sampling – Guidance on the design of sampling programs, sampling techniques and the preservation and handling of samples.
AS/NZS (Australia/New Zealand Standard) (1998). AS/NZS 5667.5:1998 Water quality – Sampling – Guidance on sampling of drinking water and water used for food and beverage processing.
AS/NZS (Australia/New Zealand Standard) (1998). AS/NZS 5667.11:1998 Water quality-Sampling – Guidance on sampling of groundwaters.
ASTM (ASTM International) (2017). D7283 Standard Test Method for Alpha and Beta Activity in Water by Liquid Scintillation Counting.
Calmet D, Ameon R, Bombard A, Forte M, Fournier M, Herranz M, Jerome S, Kwakman P, Llaurado M, Tokonami S (2013). ISO standards on test methods for water radioactivity monitoring. Applied Radiation and Isotopes, 81: 21-25.
ICRP (International Commission on Radiological Protection) (1999). Protection of the Public in Situations of Prolonged Radiation Exposure. ICRP Publication 82. Ann. ICRP 29 (1-2).
ISO (International Organization for Standardization) 9696 (2017). Water quality – measurement of gross alpha activity in non-saline water – Test method using thick source. International Standard ISO 9696. Geneva, Switzerland.
ISO (International Organization for Standardization) 9697 (2015). Water quality – measurement of gross beta activity in non-saline water – test method using thick source. International Standard ISO 9697. Geneva, Switzerland.
ISO (International Organization for Standardization) 11704 (2010). Water quality – measurement of gross alpha and beta activity concentration in non-saline water-Liquid scintillation counting method. International Standard ISO 11704. Geneva, Switzerland.
Kleinschmidt R, Black J, Akber R (2011). Mapping radioactivity in groundwater to identify elevated exposure in remote and rural communities. Journal of Environmental Radioactivity, 102: 235-243.
UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) (2000). Sources, effects and risks of ionising radiation. Report to the General Assembly. ISBN 92-1-142238-8, New York.
Walsh M, Wallner G, Jennings P (2014). Radioactivity in drinking water supplies in Western Australia. Journal of Environmental Radioactivity, 130: 56-62.
WHO (World Health Organization) (2017). Guidelines for drinking-water quality: fourth edition incorporating the first addendum. Geneva. ISBN 978-92-4-154995-0.
WHO (World Health Organization) (2018). Management of radioactivity in drinking water (2018). Geneva. ISBN 978-92-4-151374-6.
² Extract from WHO’s Management of Radioactivity in Drinking-water (WHO 2018). An operational dose value (IDC) of “0.1 mSv/year is appropriate for most countries where groundwater supplies with elevated levels of naturally occurring radionuclides are not present. Where there are elevated levels of naturally occurring radioactivity in groundwater and minimal options for alternative water sources or water treatment, a value higher than 0.1 mSv/year, but generally less than the BSS (IAEA 2014) reference level of 1 mSv/year, may be appropriate for the affected population groups.”